Force
Spectroscopy
Besides imaging
surfaces, the AFM can be also applied to study
interaction forces between tip and sample. In this case, the deflection of the
cantilever is measured as the distance between the probe and the sample is
varied, thus generating force-distance curves. Such working
configuration is called force spectroscopy. With these so-called force-distance
curves it is possible to measure intermolecular forces: by using a measuring
tip with receptors bound and recording force
vs. distance cycles on surface-bound ligands,
unbinding forces of ligand-receptor pairs can be probed at the level of single
molecule.

Recognition events
between the partners have been observed, as revealed by characteristic pull-off
jumps in the force-distance retraction curve.
By this technique
it is also possible to investigate the complex dissociation kinetics, by
monitoring the unbinding forces as a function of the scan rate.
We have used force
spectroscopy to study the interaction between redox partners, as Cytochrome C551 and Azurin, to
investigate the binding between the tumor suppressor protein p53 and the bacterial protein Azurin,
and eventually to study the interaction between the p53 and its domains ans the anticancer azurin-derived peptide p28.
Azurin-Cytochrome complex has been
studied by coupling Cytochrome C551 to the tip via a
long flexible spacer molecule, and by directly immobilizing Azurin on a flat
gold substrate, thanks to the presence of exposed cysteine
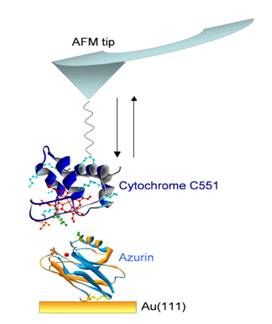 residues, via the
formation of S-Au bonds [Bonanni et al., Biophysical
Journal 2005]. Subsequently, the molecular recognition between these two
proteins has been examined after optimizing Azurin adsorption on gold via sulfhydryl terminated alkanethiol
spacers [Bonanni et al., Journal of Physical
Chemistry B 2006]. The introduction of this linker demonstrated to favour the
complex formation, as evidenced by the decreasing of the dissociation rate
constant, from 14 s-1 (for Azurin directly adsorbed on gold) to 6.7
s-1.
residues, via the
formation of S-Au bonds [Bonanni et al., Biophysical
Journal 2005]. Subsequently, the molecular recognition between these two
proteins has been examined after optimizing Azurin adsorption on gold via sulfhydryl terminated alkanethiol
spacers [Bonanni et al., Journal of Physical
Chemistry B 2006]. The introduction of this linker demonstrated to favour the
complex formation, as evidenced by the decreasing of the dissociation rate
constant, from 14 s-1 (for Azurin directly adsorbed on gold) to 6.7
s-1.
Our results, obtained
from the analysis of single electron-transfer proteins, immobilized on a metal
substrate upon two different techniques, are good starting points for possible
detection of single recognition events as an electric signal, with potential
applications in ultra-sensitive bio-nanodevices
designed for biological screening.
We have focused
our attention to the study of the p53/azurin complex. Indeed the understanding
of the molecular mechanisms, determining p53 stabilization further to Azurin
binding, may be useful for developing new targeted anticancer strategies based
on this complex formation.
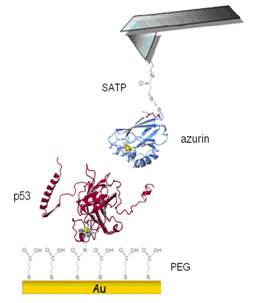
p53-Azurin complex has been
investigated by immobilizing p53 on a gold surface via a flexible poly(ethylene
glycol) (PEG) linker, and by tethering Azurin to the tip via a sulfhydryl terminated spacer. This work allowed us to
observe recognition events between the proteins and to establish that the
complex is substantially stable, displaying a lifetime of about 11 s.
p53-Mdm2-Azurin competitive experiments
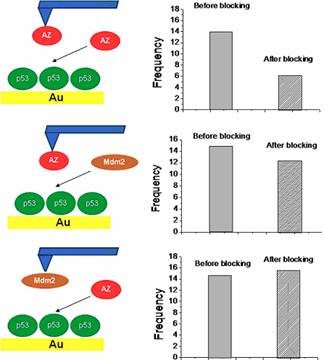 It has been demonstrated that azurin anticancer
activity is connected with its interaction with p53 that leads to both the
stabilization and intracellular level rise of the transcription factor. We have
used AFS to explore the appealing hypothesis that azurin
could compete with the main down-regulator of p53, the oncoprotein
Mdm2, for the same p53 binding site thus reducing its activity. We first measured the unbinding
frequency between a p53-functionalized substrate and an azurin-functionalized
tip and then blocked the substrate with an azurin solution. A dramatic
reduction of the unbinding frequency has been observed, confirming the
specificity of the p53/azurin interaction. Parallel, we incubated the p53
sample with a solution containing Mdm2 molecules and observed that the
frequency of interaction remained substantially unchanged. We thus performed a
second blocking experiment: we measured the unbinding frequency between
immobilized p53 and Mdm2 and then blocked the substrate with Azurin. Again, the
unbinding frequency between p53 and Mdm2 was not affected by Azurin. We thus
advanced the hypothesis that azurin and Mdm2 interact with two different
regions of p53 and that a ternary complex is possible. The occurrence of a
p53-Mdm2-azurin complex has opened a possible new scenario for the anticancer
action of azurin by also providing evidence that AFS
offers a valid tool for the study ternary complexes at single-molecule level.
It has been demonstrated that azurin anticancer
activity is connected with its interaction with p53 that leads to both the
stabilization and intracellular level rise of the transcription factor. We have
used AFS to explore the appealing hypothesis that azurin
could compete with the main down-regulator of p53, the oncoprotein
Mdm2, for the same p53 binding site thus reducing its activity. We first measured the unbinding
frequency between a p53-functionalized substrate and an azurin-functionalized
tip and then blocked the substrate with an azurin solution. A dramatic
reduction of the unbinding frequency has been observed, confirming the
specificity of the p53/azurin interaction. Parallel, we incubated the p53
sample with a solution containing Mdm2 molecules and observed that the
frequency of interaction remained substantially unchanged. We thus performed a
second blocking experiment: we measured the unbinding frequency between
immobilized p53 and Mdm2 and then blocked the substrate with Azurin. Again, the
unbinding frequency between p53 and Mdm2 was not affected by Azurin. We thus
advanced the hypothesis that azurin and Mdm2 interact with two different
regions of p53 and that a ternary complex is possible. The occurrence of a
p53-Mdm2-azurin complex has opened a possible new scenario for the anticancer
action of azurin by also providing evidence that AFS
offers a valid tool for the study ternary complexes at single-molecule level.
p53 - p28
and p53 fragments/p28 complexes have
been investigated by immobilizing p53 or its fragments on glass substrates via specific
spacer and anchoring p28 to the tip via a PEG linker. This work allowed us to
observe specific biorecognition process between p28
and both p53 and its core domain and to found that the p53 core domain/p28
complex is ten times more stable than the p28/p53 one with its 80 s half life.
With this last work we have
thus given a very significant contribution in the research on the possible
mechanism of action of the very new anticancer peptide p28.
If AFS allows the direct determination of the unbinding force
of the biorecogniton process and of its dissociation
rate constant (koff), to completely elucidate the kinetic of a biorecognition events, a Surface
Plasmons Resonance apparatus can be used.