Electron Paramagnetic Resonance
Our group has a large experience on the Electron Paramagnetic Resonance (EPR) spectroscopy. In recent years, we have applied this technique to the study of the analogy between proteins and amorphous systems. The similarity displayed in the EPR spectra at low temperature of different metallo-proteins and metallo-containing amorphous matrix was traced back to the structural heterogeneity around the paramagnetic probe; such a heterogeneity has been put into relationship to the existence of many, thermally activated local minima in the potential energy, usually called conformational substates (CS) for proteins. In addition, our approach has allowed to put into evidence that the addition of some cosolvent, like glycerol, to the protein solutions, is able to significantly reduce the structural heterogeneity of these systems.
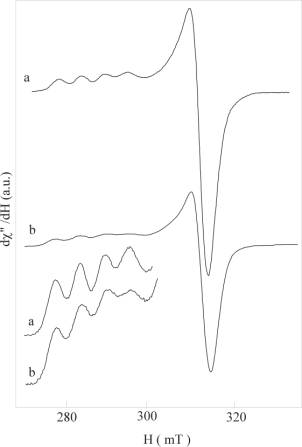
Such an analysis, first performed on myoglobin and hemoglobin, has been more recently extended to some copper containing proteins (plastocyanin and azurin): the figure shows the EPR spectra of wild type plastocyanin (a) and mutated plastocyanin containing a disulphide bridge (b). The spectral features are consistent with a typical entatic copper site geometry.