Scanning Probe Microscopy
The very high resolution reachable by Scanning Probe Microscopy allows the study of structural (topographic) and functional (electronic conduction) properties at the level of a single molecules, as a function of the environment conditions.
Actually, several redox metalloproteins (azurin, engineered plastocyanin, cytochrome c) self-chemisorbed onto Au(111) substrates are under investigation by SPM techniques (scanning force/lateral force microscopy, current sensing AFM, scanning tunnelling spectroscopy, electrochemically controlled scanning tunnelling microscopy (Ultramicroscopy, 2001)) in wet and dry environments.
Our activity is currently focused on the following topics.
Topographical characterization of single protein
The combination of Atomic Force Microscopy (AFM) and Scanning Tunnelling Microscopy (STM) allows a topographical characterisation of the adsorbed proteins at the level of single molecule, providing information about how proteins are immobilised on the electrode surface and how immobilisation may affect their structure.
Tapping Mode Atomic Force Microscopy (TMAFM) appears to be a helpful tool to image self-assembled biomolecules at the single molecule level. Indeed, such technique is focused on reducing the lateral force applied to biomolecules by periodically etappingf the sample surface with the tip. Representative TMAFM images of azurin and PCSS adsorbed on Au(111) substrate in buffer solution are shown below.
Azurin PCSS
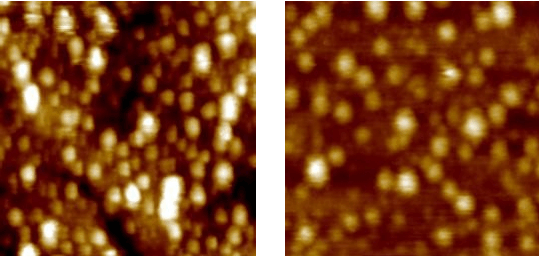
400 nm 350 nm
Individual molecules can be resolved above the gold substrate, as shown in the higher resolution image below:
Azurin
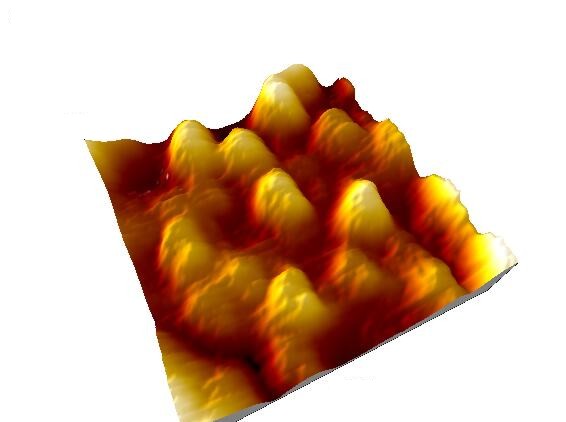
130 nm
TMAFM provides information about morphological properties of adsorbed single molecules. In particular, the analysis of cross section profile over several individual molecules give the molecular height distribution also providing information about protein orientation above the substrate.
PCSS
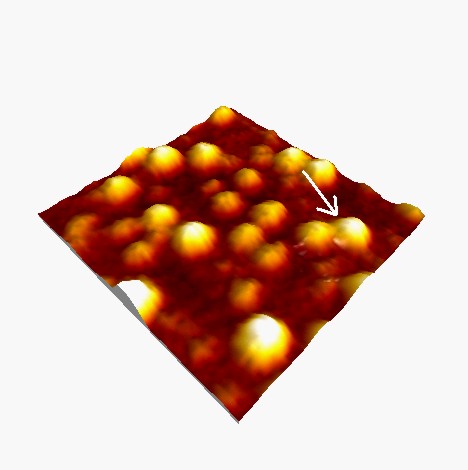
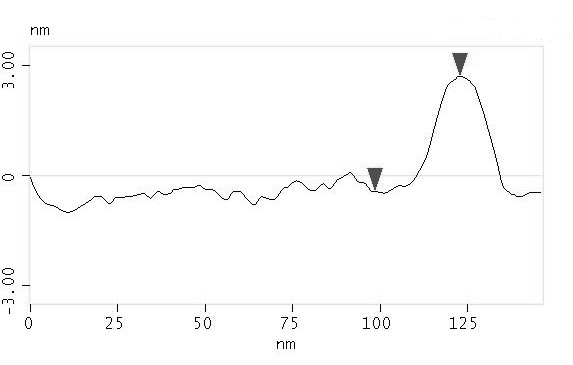
145 nm
The statistical analysis of PCSS molecular heights, estimated from hundreds individual cross sections, shows that the data can be approximated by a normal distribution centered at 2.3 nm, with a standard deviation of 0.5 nm.
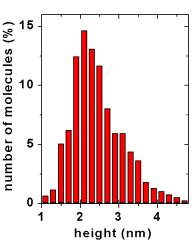
The single mode distribution is indicative of a preferential orientation of the proteins above the substrate, as expected for site-preferential adsorption. Moreover, the mean value close to the crystallographic data is consistent with a non denaturing adsorption (Surface Science, 2003).
The typical protein lateral dimension, as evaluated from full width at half maximum of molecule cross section profile, is considerably larger than the crystallographic data, due to the well-known tip broadening effect.
However, protein lateral dimension can be generally recovered by STM imaging which is known to induce a minor tip convolution respect to AFM, often revealing interesting submolecular features.
Yeast Cytochrome C molecules adsorbed on gold substrate have been imaged by in situ STM. A typical STM image recorded in liquid is here shown.
YCC
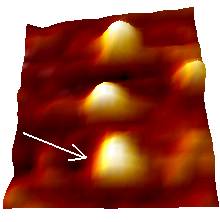
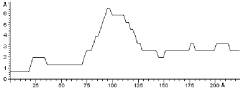
24.5 nm
The lateral dimensions are in good agreement with the crystallographic data, whereas the apparent height is reduced. Indeed, STM images are a complex convolution of structural and electronic contributions, so that the height data may deviate significantly from the purely topographic one.
Despite the true nature of conduction around/through bio-molecules, one of the major problems remains how to quantify the tip to sample distance, and how this parameter can affect the measure. In fact, estimating the distance between the STM probe and the sample surface remains a key requirement to decide whether the STM method is invasive or not.
At this purpose, the dependence of the tunnelling current flowing between the STM probe and a metal surface and the relative tunnelling gap is investigated in different media. The distance corresponding to a selected tunnelling resistance was obtained after evaluation of the inverse decay length K= -1/2(dlnIT/ds), where s is the gap between tip and sample.
If the tunnelling resistance is set to 4 x 10 9 ohms, distances of 3 nm and up to 6 nm were estimated when Au electrodes are imaged in water and in air, respectively.
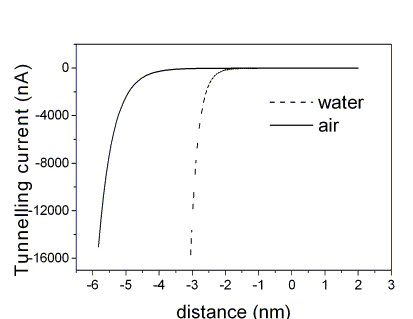
Based on this result, tunnelling distances between tip and single globular-proteins on a flat surface have been revisited in the case of Plastocyanin chemi-sorbed on Au(111) electrodes.
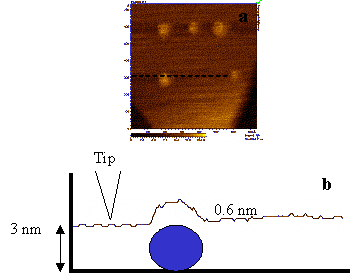
PCSS on Au(111) under liquid
Electronic conduction through/around biomolecules by STS and CSAFM
Since the prosthetic groups of many important proteins play a key role in electron transfer activities, conductivity through Fe-protoporphyrin IX (FePP) is studied by STM. Scanning Tunnelling Spectroscopy (STS) is here used to monitor the tunnelling properties of FePP by altering the total potential difference between the substrate and the tip (Vbias) and measuring the resulting currents ITunnel.
FePP is induced to self-assemble under electrochemical control on Highly Oriented Pyrolitic Graphite (HOPG), therefore electron transfer through the monolayer of molecules is probed by recording ITunnel as a function of the bias Vbias between tip and substrate. To focus the attention on temporal fluctuation exhibited by ITunnel vs. Vbias spectra, the current flowing around/through a single FePP is also recorded by directly adsorbing it on STM tip made from Silver.
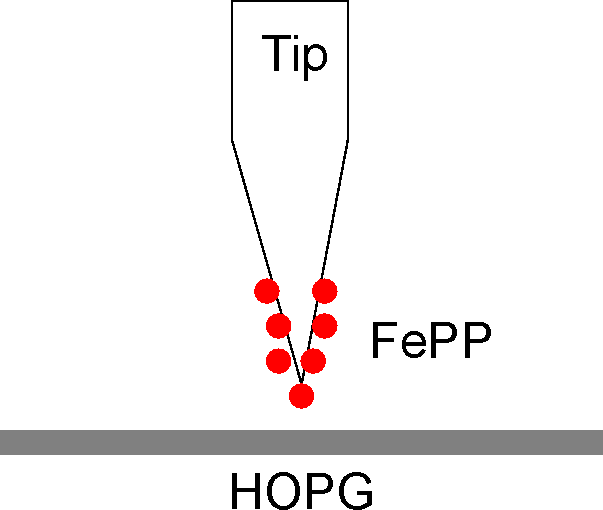
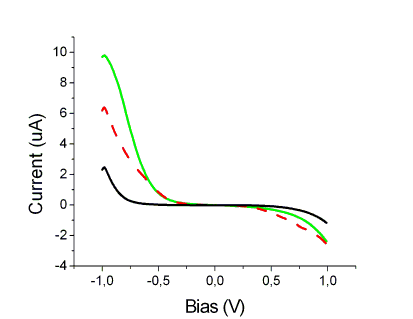
Experimental setup vs. Vbias of FePP adsorbed on Ag Tip
A possible correlation with temporal fluctuations recently observed in Surface enhanced resonance Raman (SERRS) spectra (Chemical Physics, 2003) is under investigation.
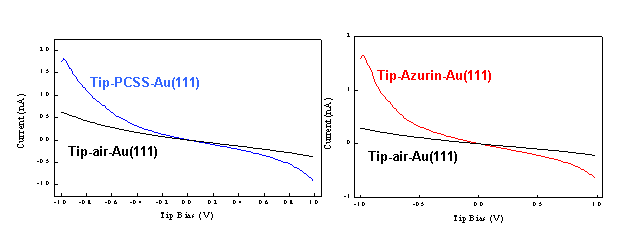
A similar approach has been used to investigate th electronic conduction through single metallo-proteins. More in details, the electronic properties of PCSS mutant and wild type azurin were investigated by using scanning tunneling spectroscopy (STS) in ambient conditions. The STS measurements, acquired by positioning the STM tip over single molecules, show a considerable asymmetry in the I-V curves, pointing out a possible rectifying behaviour of copper proteins.
|
|
Current sensing atomic force microscopy (CSAFM) simultaneously probes topography, friction and conductivity by controlling the relative pressure, i.e. distance, between conductive tip and sample.
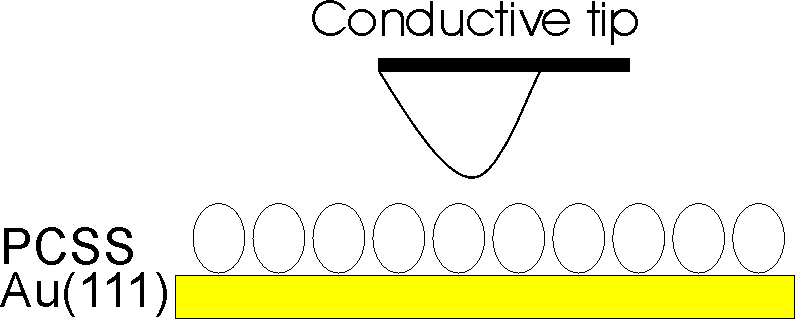
Sketch of CSAFM technique.
In this project, CSAFM is explored as a tool for investigating conductivity around/through bio-molecules as a function of the pressure of the scanning tip and the environmental conditions.
More in details, engineered plastocyanin (PCSS) proteins were chemi-sorbed on Au(111) and characterized by recording I vs VBias spectra by CSAFM in contact regime at different applied forces.
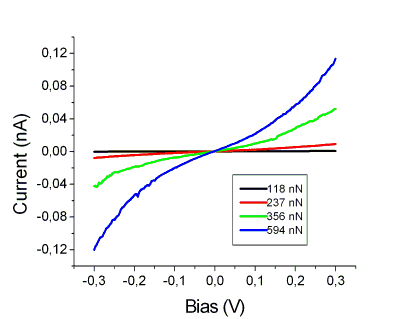
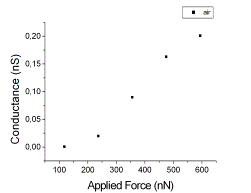
Back to Single Molecule Spectroscopy